- The Ultimate Guide to Metallurgical Testing: Everything You Need to Know
- The Lead Content in Soil Near Notre Dame after the Fire Is 65 Times Higher than the Reference Value
- Surfactant Analysis by HPLC: A Comprehensive Guide
- Italian Fake Olive Oil Flooded into the Market - 24 Suspects Were Arrested by the Police
- A Comprehensive Guide to Cosmetic Stability Testing Guidelines
- Understanding Food Nutrition Labels
- Home
-
Industries
-
Environment Testing
- Construction Dust Testing
- Drinking Water Testing
- Emerging Contaminants Testing
- Sea Water Testing
- Sludge Testing
- Soil Testing
- Solid Waste
- Stack Emissions Testing
- Stormwater Testing
- Subsurface Vapour Intrusion Assessment
- Surface Water Testing
- Wastewater Testing
- Watersheds and Rivers Testing
- Well Water Testing
- Asbestos Testing
- Mold Testing
-
Food Testing
- Beverage Testing
- Biscuit Testing
- Candy Testing
- Canned Food Testing
- Coffee Testing
- Condiments Testing
- Cooking Oil Testing
- Fast Food and Snack Testing
- Infant Food Testing
- Liquor Testing
- Meat and Meat Product Testing
- Milk & Dairy Testing
- Pet Food Testing
- Refrigerated and Frozen Food Testing
- Seafood Testing
- Tomato Products Testing
- Agricultural Products & Crops Testing
- Material Testing
- Chemical Products Testing
- Petroleum Products Testing
- Personal Care & Beauty Products Testing
- Household and Apparel Products Testing
- Healthcare Products Testing
- Building Products Testing
- Stationery and Office Supplies Testing
- Safety Testing of Nano Products
- Children Products Testing
-
Environment Testing
-
Services
- Agriculture & Crop Analytical Services
- Energy Analytical Services
- Environmental Analytical Services
- Food Analytical Services
- Material Characterization Services
-
Pharmaceutical Analytical Services
- Biopharmaceutical Characterization Services
- Deformulation (Reverse Engineering) Analysis Services
- Pharmaceutical and Medical Device Failure Analysis Services
- Pharmaceutical Impurity Testing Services
- Pharmaceutical Separation and Purification Services
- Preformulation Analysis Services
- Stability Analysis Services
- Compendial Testing
- Dissolution Testing
- Water Content Determination
- Pharmaceutical Water Testing
- Potency Testing
- Pharmaceutical Microbiology Testing
- Retail Products Analytical Services
- Textile Testing Services
- Karl Fischer (KF) Moisture Testing
-
Recommended Services
- Failure Analysis and Investigations
- Food Testing
- Pharmaceutical Testing
- Surfactant Testing
- Cosmetics and Skin Care Products Testing
- Karl Fischer (KF) Moisture Testing Service
- Textile Testing
- Microplastic Analysis and Testing
- Mold Testing Service
- Lubricating Oil Analysis and Testing
- Alloy Material Testing
- Techniques
- AI SmartQC Platform
-
Resources
- Regulatory Resources
- Blog
- Application Notes
- Video Library
- White Paper
- Flyer
- Case Study
- Fee Schedule
-
Protocol
- Protocol for Deformulation of Pharmaceutical Products
- Experimental Procedure for Determining Trace Moisture in Food by Karl Fischer Method
- Experimental Procedure for Determining Moisture Content in Interior Wall Paint Using Karl Fischer Method
- Testing of Color Fastness to Rubbing for Textiles
- Qualitative Identification of Textile Fibers by Five Experimental Methods
- Tablet Dissolution Test - Determination of Dissolution Rate and Dissolution Rate of Azithromycin Dispersible Tablets
- Determination of Chemical Oxygen Demand (COD) in Water - Potassium Dichromate Method
- How to Detect Bacteria in Food: Total Bacterial Count Procedure
- Testing Coliforms in Food: MPN & Plate Count Methods
- Determination of Aflatoxin B1 in Food: ELISA & TLC Methods for Accurate Detection
- Determination of Molds and Yeasts in Food: Plate Count Methods for Accurate Detection
- Determination of Lubricating Oil Liquid Density - Density Meter Method
- Determination of Acid Value in Lubricating Oil - Potentiometric Titration Method
- ELISA Experimental Procedure for Detecting Aflatoxin B1 in Peanuts
- Careers
- About
- Contact
- Home
- Resources
- Blog
- Healthcare Products
- Vaccine Safety and Immunogenicity Analysis
Vaccine Safety and Immunogenicity Analysis
InquiryThrough our global network of testing experts and analytical equipment including chromatography (HPLC, GC, GC/MS) and atomic absorption spectroscopy (AAS, GFA, FIAS), Our goal is to provide test services as efficiently as possible to maximize our customers' profits. For more information about our services, contact one of our experts today.
Note: this service is for Research Use Only and Not intended for clinical use.
Vaccine is an autoimmune preparation for preventing infectious diseases by artificially attenuating, inactivating or using genetic engineering methods for pathogenic microorganisms (such as bacteria, rickettsia, viruses, etc.) and their metabolites. The vaccine retains the characteristics of the pathogen to stimulate the immune system of the animal. The vaccine has three basic properties: safety, immunogenicity and stability.
Vaccine and Vaccination
Vaccination is a procedure in which the immune system is exposed to an antigen, such as an inactivated toxin or attenuated pathogen, to elicit antigen-specific clonal expansion (i.e., the proliferation of T cells and B cells that recognize the antigen), which aims to help the body to defend itself. A vaccine against infection is a modified form of a natural immunogen, which may be either the whole pathogen, one of its components, or a toxin. A vaccine does not cause disease when administered but induces the healthy host (the vaccinee) to mount a primary response against epitopes of the modified immunogen and to generate large numbers of memory B and T cells. In a vaccinated individual, a collection of circulating antibodies and an expanded army of pathogen-specific memory B and T cells have already been generated prior to a first exposure to the natural pathogen. When the natural pathogen attacks, the circulating antibodies provide a degree of immediate protection from the invader. This type of vaccination is called prophylactic vaccination because it is intended to prevent disease.
Analysis of Vaccine Safety
Every licensed and recommended vaccine goes through years of safety testing including: (1)Testing and evaluation of the vaccine before it’s licensed by the Food and Drug Administration (FDA) and recommended for use by the Centers for Disease Control and Prevention (CDC). (2)Monitoring the vaccine’s safety after it is recommended for infants, children, or adults.
Analysis of vaccine characteristics
Vaccines include inactivated vaccines, genetically engineered vaccines, and live attenuated vaccines. The inactivated vaccine and the genetically engineered vaccine have relatively simple components, mainly purified protein and polysaccharide components, which have high purity and are relatively stable; the live attenuated vaccine consists of attenuated vaccine strains. According to the composition and physical and chemical properties of the vaccine, in the case of complete packaging, the cold chain will be removed in a short period of time, and no toxic substances will be produced. The World Health Organization has also repeatedly pointed out that vaccines that are released from cold chain storage in the short term will hardly cause toxicity.
Effect analysis of vaccine
1). Animal model detection
Animal models can be used not only to detect the pathological, genetic, and molecular biological mechanisms of vaccine molecules, but also to test their safety, toxicity, and effects, such as toxicity tests, virulence tests, and protective immunity tests, etc. In addition, transgenic animals can also be used to identify pathological responses and adjuvant effector mechanisms caused by vaccination. The following tests were performed once the animal experiment was determined to be safe and effective.
2). A small number of people with immunological effects and side effects
That is, clinical trials (stages I to IV) include a series of identification processes such as vaccine safety, side effects, immunogenicity, and protective immune response.
Methods: Mentioned in the Reisinger KS experiment, in this randomized, double-blind trial, 1781 sexually naive children were assigned (2:1) to quadrivalent HPV-6/11/16/18 vaccine or saline placebo administered at day 1 and months 2 and 6. Serum neutralizing anti-HPV-6/11/16/18 responses were summarized as geometric mean titers (GMTs) and seroconversion rates. Primary analyses were done per-protocol (subjects received 3 doses, had no major protocol violations and were HPV type-specific seronegative at day 1). Adverse experiences were collected by diary card. Results are as follows:

Figure.1 Vaccine safety randomized trial6
3). Epidemiological effect evaluation
The epidemiological effect evaluation is to study the changes in the epidemiological characteristics of the disease after vaccination in order to evaluate the effect of the vaccine. The subjects and groups of the experiment (including the control group) should follow the random and double-blind principle to compare the differences between the immunized and the unimmunized population, and compare the prevalence of the disease in the control group and the immunized group in a specific period; for the observation of several epidemic cycles for many years and years, analyze the epidemiology of the disease to evaluate the impact of the vaccine on the epidemic after a large-scale use. In addition, the incidence of adverse reactions after immunization of local residents should be correctly assessed, the contraindications and benefits/risk of the vaccine should be analyzed, and the ethical problems in the use of vaccines should be established to establish an after-sales monitoring system to strengthen the monitoring of vaccine quality. And making the improvements so that the quality and effectiveness of vaccines are continuously optimized and improved.
Analysis of thermal stability of the vaccine
Vaccines, as biological products, are temperature sensitive and need to be stored and transported under cold chain conditions to maximize and ensure vaccine effectiveness. The thermal stability of vaccines is a key indicator of vaccine development, preparation, and validation. In the process of vaccine development and preparation, measures such as adding a protective agent and a freeze-drying process are adopted to improve the stability and protect the efficacy of the vaccine.
The thermal stability of the vaccine is effectively guaranteed during production and verification. In order to ensure the stability of the vaccine in the actual storage and use environment, the rigorous long-term stability test is carried out at the time of approval of the vaccine product. Generally, the effective period of the product is determined based on the reduction of the actual measurement stability time by 6 months. For heat-sensitive vaccines, each batch of vaccines must be subjected to a thermal acceleration stability test (also called “hallenge test”) at 37 ° C for 1 to 4 weeks before leaving the factory.
Analysis of vaccine Immunogenicity
Immunogenicity refers to the ability to stimulate the body to form specific antibodies or sensitize lymphocytes. That is to say, the antigen can stimulate specific immune cells, activate, proliferate, and differentiate immune cells, and finally produce antibodies to immune effector antibodies and sensitized lymphocytes. The WHO stipulates that after the vaccination, the neutralizing antibody induced ≥0.5 IU/ml can provide sufficient immune protection.
Protein drugs are potentially immunogenic and may induce anti-Drug Antibodies (ADAs). ADAs may reduce the efficacy of drugs, or cause allergic reactions, and even life-threatening. The study of the correlation between immunogenicity and clinical symptoms relies on the objective detection and characterization of anti-drug antibodies in preclinical and clinical studies.
Evaluation methods
1). In silico screening.
The T cell epitope content can now be measured relatively accurately using computer tools, which is one of the factors leading to the risk of immunogenicity. Immunoinformatics algorithms for identifying T cell epitopes are now being applied to classify protein therapeutics into higher risk and lower risk categories. Using this method, the clinical immunogenicity of novel protein therapeutics can be calculated.
Methods: Flow measurement, the detection parameters are set according to the fluorescent substances labeled by the surface markers of each T lymphocyte in the flow detection antibody mix, and the upflow analyzer FACS canto ‖ is used for measurement.
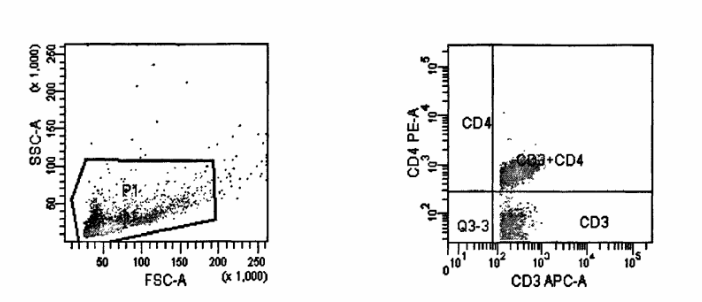
Figure.2 Flow analysis CD3+,CD4+ cell7
2). Enzyme-Linked Immunosorbent Assay(ELISA)
The collected serum samples were tested for specific antibodies by ELISA, and the geometric mean titer was calculated.
References
Leroux-Roels G. ‘Vaccine development’. Perspectives in Vaccinology. 1: 115–150.
Eugenia M. Dragunsky Alexander P., etc. (2004) ‘Evaluation of Immunogenicity and Protective Properties of Inactivated Poliovirus Vaccines: A New Surrogate Method for Predicting Vaccine Efficacy’. Infectious Diseases, 190(8), 1404-1412.
BLACK, STEVEN MD, SHINEFIELD, HENRY MD, etc. (2000) ‘Efficacy, safety and immunogenicity of the heptavalent pneumococcal conjugate vaccine in children’. The Pediatric Infectious Disease Journal, 19(3), 187-195.
Melief C, van Hall T, Arens R, Ossendorp F, van der Burg S. (2015). ‘Therapeutic cancer vaccines’. J Clin Invest, 125 (9), 3401-3412.
Reisinger, Keith S. MD, MPH., etc. (2007) ’Safety and Persistent Immunogenicity of a Quadrivalent Human Papillomavirus Types 6, 11, 16, 18 L1 Virus-Like Particle Vaccine in Preadolescents and Adolescents: A Randomized Controlled Trial’, Vaccine, 26(3), 201-209.
Yan Ma. (2011) ‘Preparation and immunogenicity of DTaP-sIPV quadruple vaccine’, Chinese Academy of Medical Sciences.

Alfa Chemistry is a professional analytical testing company, and has long been committed to promoting the long-term development of the analytical testing industry. We sincerely welcome colleagues from all walks of life to come to negotiate and cooperate.
- Recommendation
- Ordering Process
- Ask a Question
Do not know how to place an order, please refer to the flow chart shown below.
Submit quotation request |
A technical manager will contact you within 24 hours |
You will review and approve the final price and place an order |
Confirm with you and make the payment |
Instruct you to ship your samples and form |
Analytic report delivery |

