- Home
-
Industries
-
Environment Testing
- Construction Dust Testing
- Drinking Water Testing
- Emerging Contaminants Testing
- Sea Water Testing
- Sludge Testing
- Soil Testing
- Solid Waste
- Stack Emissions Testing
- Stormwater Testing
- Subsurface Vapour Intrusion Assessment
- Surface Water Testing
- Wastewater Testing
- Watersheds and Rivers Testing
- Well Water Testing
- Asbestos Testing
- Mold Testing
-
Food Testing
- Beverage Testing
- Biscuit Testing
- Candy Testing
- Canned Food Testing
- Coffee Testing
- Condiments Testing
- Cooking Oil Testing
- Fast Food and Snack Testing
- Infant Food Testing
- Liquor Testing
- Meat and Meat Product Testing
- Milk & Dairy Testing
- Pet Food Testing
- Refrigerated and Frozen Food Testing
- Seafood Testing
- Tomato Products Testing
- Agricultural Products & Crops Testing
- Material Testing
- Chemical Products Testing
- Petroleum Products Testing
- Personal Care & Beauty Products Testing
- Household and Apparel Products Testing
- Healthcare Products Testing
- Building Products Testing
- Stationery and Office Supplies Testing
- Safety Testing of Nano Products
- Children Products Testing
-
Environment Testing
-
Services
- Agriculture & Crop Analytical Services
- Energy Analytical Services
- Environmental Analytical Services
- Food Analytical Services
- Material Characterization Services
-
Pharmaceutical Analytical Services
- Biopharmaceutical Characterization Services
- Deformulation (Reverse Engineering) Analysis Services
- Pharmaceutical and Medical Device Failure Analysis Services
- Pharmaceutical Impurity Testing Services
- Pharmaceutical Separation and Purification Services
- Preformulation Analysis Services
- Stability Analysis Services
- Compendial Testing
- Dissolution Testing
- Water Content Determination
- Pharmaceutical Water Testing
- Potency Testing
- Pharmaceutical Microbiology Testing
- Retail Products Analytical Services
- Textile Testing Services
- Karl Fischer (KF) Moisture Testing
-
Recommended Services
- Failure Analysis and Investigations
- Food Testing
- Pharmaceutical Testing
- Surfactant Testing
- Cosmetics and Skin Care Products Testing
- Karl Fischer (KF) Moisture Testing Service
- Textile Testing
- Microplastic Analysis and Testing
- Mold Testing Service
- Lubricating Oil Analysis and Testing
- Alloy Material Testing
- Deformulation Service
- Techniques
- AI SmartQC Platform
-
Resources
- Regulatory Resources
- Blog
- Application Notes
- Video Library
- White Paper
- Flyer
- Case Study
- Fee Schedule
-
Protocol
- Protocol for Deformulation of Pharmaceutical Products
- Experimental Procedure for Determining Trace Moisture in Food by Karl Fischer Method
- Experimental Procedure for Determining Moisture Content in Interior Wall Paint Using Karl Fischer Method
- Testing of Color Fastness to Rubbing for Textiles
- Qualitative Identification of Textile Fibers by Five Experimental Methods
- Tablet Dissolution Test - Determination of Dissolution Rate and Dissolution Rate of Azithromycin Dispersible Tablets
- Determination of Chemical Oxygen Demand (COD) in Water - Potassium Dichromate Method
- How to Detect Bacteria in Food: Total Bacterial Count Procedure
- Testing Coliforms in Food: MPN & Plate Count Methods
- Determination of Aflatoxin B1 in Food: ELISA & TLC Methods for Accurate Detection
- Determination of Molds and Yeasts in Food: Plate Count Methods for Accurate Detection
- Determination of Lubricating Oil Liquid Density - Density Meter Method
- Determination of Acid Value in Lubricating Oil - Potentiometric Titration Method
- ELISA Experimental Procedure for Detecting Aflatoxin B1 in Peanuts
- Careers
- About
- Contact
- Home
- Industries
- Material Testing
- Nanomaterials Testing
Nanomaterials Testing
InquiryThrough our global network of testing experts and analytical equipment including chromatography (HPLC, GC, GC/MS) and atomic absorption spectroscopy (AAS, GFA, FIAS), Our goal is to provide test services as efficiently as possible to maximize our customers' profits. For more information about our services, contact one of our experts today.
Note: this service is for Research Use Only and Not intended for clinical use.
Nanotechnology deals with structures between 1 to 100 nanometers. Nanomaterials and novel engineered nanotechnology offer great potential to improve the quality of life when used in applications across a variety of industries and consumer products, and range from computer memory storage to sunscreens. Nanomaterials currently in existence exhibit various physical, chemical, mechanical, optical, magnetic and biological properties, as well as different internal/external structures. Nanomaterials are of great scientific interest as they are, in effect, a bridge between bulk materials and atomic or molecular structures. Bulk materials usually maintain constant physical properties regardless of size, however, size-dependent properties are often observed at the nanoscale. Thus, the properties of materials change as their sizes approach the nanoscale and the surface area to volume ratio becomes significant.
As a global leading nanomaterials characterization company, Alfa Chemistry offers a strong array of capabilities and testing services to nanomaterials. From morphology analysis to crystalline phase determination, Alfa Chemistry provides incredible service and credible results. Alfa Chemistry is your one-stop-shop laboratory performing all of nanomaterials characterization.
Nanomaterials for consumer products can be characterized by us:
Textiles
Pharmaceuticals- Nanoencapsulation
Food and Nutrition
Cosmetics
Nano-dispersions
Speciality Chemicals – Such as Insecticides
Rubbers, Plastics and Composites Materials
Electronics
Processing of nano-materials
Nano-particles
Alfa Chemistry's nanomaterials characterization services include:
| Testing Items | Project Content |
|---|---|
| Morphology analysis | Geometric morphology, particle size, particle size distribution, morphology micro-area composition, phase structure, etc. |
| Size analysis | Particle size and shape, etc. |
| Structural analysis | Phase structure, crystal structure, etc. |
| Optical performance | Clarity, reflectance, refractive index, etc. |
| Mechanical property | Toughness, scratch resistance or impact strength, etc. |
| Other | Surface analysis, component analysis, polymer matrix, filler content, physical properties and processing conditions investigations, etc. |
Techniques for nanomaterials testing
Scanning Electron Microscope
The Scanning Electron Microscope (SEM) is an instrument that uses a high-energy electron beam to scan the surface of a sample and detect the generated signals to obtain information about the sample's surface morphology and composition. In nanomaterial research, SEM is commonly used to observe the morphology, distribution, and microscopic structure of nanoparticles.
Transmission Electron Microscope
The Transmission Electron Microscope (TEM) is a high-resolution microscope that uses a high-speed electron beam to pass through the sample, obtaining information about the material's morphology, crystal structure, and composition distribution. TEM plays an important role in nanomaterial research, providing very useful information for characterizing the lattice structure, particle size, and phase composition of nanomaterials.
Atomic Force Microscope
The Atomic Force Microscope (AFM) is a technique that uses a small force sensor to detect the interaction force between the sample surface and the probe, providing three-dimensional surface morphology and local force characteristics of the sample. AFM is widely applied, capable of observing atomic- and molecular-level surface structures and shapes, as well as characterizing and measuring nanoparticles.
X-ray Diffractometer
The X-ray Diffractometer (XRD) is a method that uses the diffraction of X-rays by materials to create interference phenomena from different crystal planes, thereby determining the crystal structure and composition. In nanomaterial research, XRD helps researchers obtain information about the crystal structure, grain size, and corresponding crystallinity of nanomaterials.
Raman Spectrometer
The Raman Spectrometer can induce vibrations and rotations of sample molecules using a laser, causing the frequency of laser photons to shift based on the different vibration energies, enabling the study of material's molecular structure, chemical bonds, and lattice structures. In nanomaterial research, Raman spectroscopy has unique advantages and can be used to study nanostructures, including material structure, size and distribution, and surface physicochemical properties.

Industry articles
Characterization of TiO2 Nanomaterials for Nanofluid Applications
 Kristiawan, Budi, et al. Nano-Structures & Nano-Objects 38 (2024): 101168.
Kristiawan, Budi, et al. Nano-Structures & Nano-Objects 38 (2024): 101168.
This study investigates the structural properties of TiO2 nanoparticles in water-ethylene glycol (MEG-DW) based nanofluids, focusing on crystallite size, microstrain, and phase determination. Using a two-step process, TiO2 nanoparticles were dispersed in MEG-DW mixtures at varying concentrations (10:90, 25:75, 40:60) to form TiO2-3%/MEG nanofluids. The crystallite size was analyzed using the Scherrer equation, Williamson–Hall (W–H) plot, and TEM-ImageJ software, yielding sizes of 27.75, 28.82, and 29.80 nm for the respective nanofluids. TEM analysis further confirmed a nanoparticle size of approximately 39.6 nm in MEG-DW suspensions.
The W–H plot method provided additional insights, revealing microstrain values between 0.000020 and 0.001386, indicating minimal strain within the nanoparticles. XRD and TEM confirmed that the TiO2 nanoparticles exhibited a rutile phase, a structure known for its high-temperature stability. This stability suggests that TiO2-based nanofluids could be viable for use in industrial heating systems where high thermal resistance is essential.
The findings provide a comprehensive understanding of TiO2 nanoparticle characteristics, demonstrating their potential for diverse applications, especially in thermal management and fluid-based technologies. The combination of XRD and TEM analysis is emphasized as an effective approach to nanomaterial characterization.
Co-catalytic Performance and Characterization of WS2 Nanomaterials in PMS Activation
 Ren, Xuechang, et al. Journal of Environmental Chemical Engineering 12.5 (2024): 114082.
Ren, Xuechang, et al. Journal of Environmental Chemical Engineering 12.5 (2024): 114082.
This study explores the role of tungsten disulfide (WS2) nanomaterials as effective co-catalysts in activating peroxymonosulfate (PMS) for the degradation of organic pollutants. Three WS2 samples with varying morphologies, specific surface areas, and 1T phase contents were synthesized and characterized using SEM, TEM, BET, XRD, Raman, and XPS. The results showed that the a-WS2 sample exhibited the largest specific surface area, the highest 1T phase content, and superior co-catalytic performance compared to other WS2 variants.
The a-WS2/Fe(II)/PMS system effectively degraded 20 mg/L phenol within 15 minutes and also demonstrated catalytic efficiency in removing RhB (Rhodamine B) and OFX (Ofloxacin). The study identified ten intermediate products during phenol degradation, providing insight into the transformation pathway. Mechanistic analysis revealed that WS2 facilitated the conversion of Fe(III) to Fe(II), enhancing PMS activation. The active radicals responsible for degradation included ·SO4−, ·OH, and ·O2−.
The a-WS2 system demonstrated excellent stability, recyclability, and a low ion leaching rate, with sulfur vacancies further enhancing its co-catalytic activity. These findings highlight WS2 nanomaterials as a promising catalyst for advanced wastewater treatment, particularly for high-concentration organic pollutants. The study provides a deeper understanding of WS2's role in PMS-based catalytic degradation, supporting its potential for industrial and environmental applications.
Surface Characterization of Covalently Functionalized Carbon-based Nanomaterials
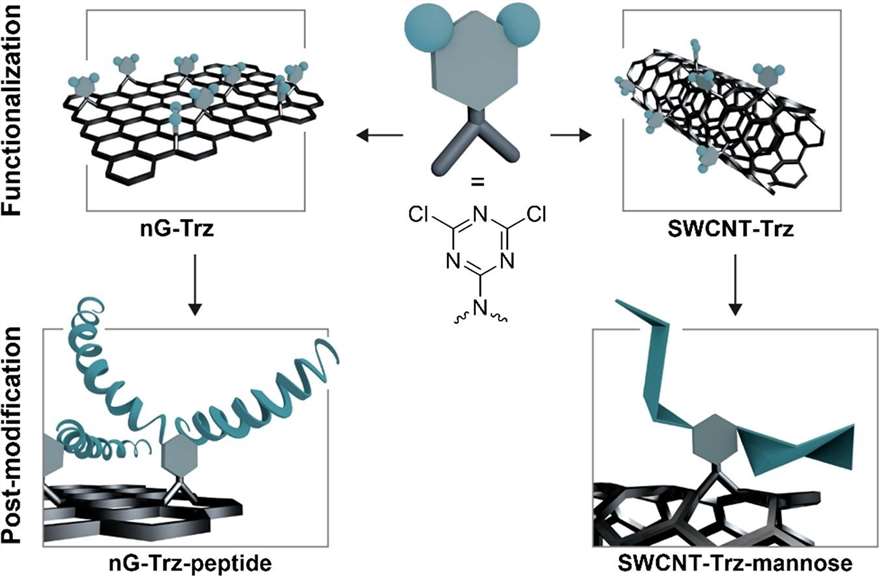 Nickl, Philip, et al. Applied Surface Science 613 (2023): 155953.
Nickl, Philip, et al. Applied Surface Science 613 (2023): 155953.
Accurate characterization of carbon-based nanomaterials at the atomic level is critical for exploring their functionalization and potential applications. This study employs a combination of x-ray photoelectron (XP) spectroscopy and near edge x-ray absorption fine structure spectroscopy (NEXAFS) to investigate the covalent functionalization of single-walled carbon nanotubes (SWCNT) and nanographene (nG) through nitrene [2+1]-cycloaddition with electron-poor monoazido-dichloro-triazine. The analysis confirms that the π-conjugated system, essential for the aromaticity of the materials, is preserved after functionalization, which is challenging to demonstrate using XP spectroscopy alone.
The comprehensive use of XP and NEXAFS techniques allowed for precise quantification of the functionalization degree and detailed analysis of the carbon-carbon bonding, specifically Csp2 proportions before and after the modification. Post-functionalization, both nG and SWCNT showed similar structural integrity, confirming the utility of this approach for analyzing the covalent modification of nanomaterials.
This methodology offers a robust framework for investigating complex functionalization processes at the atomic level. The findings are significant for future applications in creating targeted nanomaterials, particularly for interactions with biological systems such as protein targeting ligands, expanding the potential of functionalized carbon-based nanomaterials in a wide range of technological and biomedical fields.
Nanomaterials test guidance, standards and specifications
ISO
ASTM
- Regulatory Resources
- Application Notes
- Blog
- Recommendation
- Ordering Process
- Ask a Question
Do not know how to place an order, please refer to the flow chart shown below.
Submit quotation request |
A technical manager will contact you within 24 hours |
You will review and approve the final price and place an order |
Confirm with you and make the payment |
Instruct you to ship your samples and form |
Analytic report delivery |


