- Home
-
Industries
-
Environment Testing
- Construction Dust Testing
- Drinking Water Testing
- Emerging Contaminants Testing
- Sea Water Testing
- Sludge Testing
- Soil Testing
- Solid Waste
- Stack Emissions Testing
- Stormwater Testing
- Subsurface Vapour Intrusion Assessment
- Surface Water Testing
- Wastewater Testing
- Watersheds and Rivers Testing
- Well Water Testing
- Asbestos Testing
- Mold Testing
-
Food Testing
- Beverage Testing
- Biscuit Testing
- Candy Testing
- Canned Food Testing
- Coffee Testing
- Condiments Testing
- Cooking Oil Testing
- Fast Food and Snack Testing
- Infant Food Testing
- Liquor Testing
- Meat and Meat Product Testing
- Milk & Dairy Testing
- Pet Food Testing
- Refrigerated and Frozen Food Testing
- Seafood Testing
- Tomato Products Testing
- Agricultural Products & Crops Testing
- Material Testing
- Chemical Products Testing
- Petroleum Products Testing
- Personal Care & Beauty Products Testing
- Household and Apparel Products Testing
- Healthcare Products Testing
- Building Products Testing
- Stationery and Office Supplies Testing
- Safety Testing of Nano Products
- Children Products Testing
-
Environment Testing
-
Services
- Agriculture & Crop Analytical Services
- Energy Analytical Services
- Environmental Analytical Services
- Food Analytical Services
- Material Characterization Services
-
Pharmaceutical Analytical Services
- Biopharmaceutical Characterization Services
- Deformulation (Reverse Engineering) Analysis Services
- Pharmaceutical and Medical Device Failure Analysis Services
- Pharmaceutical Impurity Testing Services
- Pharmaceutical Separation and Purification Services
- Preformulation Analysis Services
- Stability Analysis Services
- Compendial Testing
- Dissolution Testing
- Water Content Determination
- Pharmaceutical Water Testing
- Potency Testing
- Pharmaceutical Microbiology Testing
- Retail Products Analytical Services
- Textile Testing Services
- Karl Fischer (KF) Moisture Testing
-
Recommended Services
- Failure Analysis and Investigations
- Food Testing
- Pharmaceutical Testing
- Surfactant Testing
- Cosmetics and Skin Care Products Testing
- Karl Fischer (KF) Moisture Testing Service
- Textile Testing
- Microplastic Analysis and Testing
- Mold Testing Service
- Lubricating Oil Analysis and Testing
- Alloy Material Testing
- Deformulation Service
- Techniques
- AI SmartQC Platform
-
Resources
- Regulatory Resources
- Blog
- Application Notes
- Video Library
- White Paper
- Flyer
- Case Study
- Fee Schedule
-
Protocol
- Protocol for Deformulation of Pharmaceutical Products
- Experimental Procedure for Determining Trace Moisture in Food by Karl Fischer Method
- Experimental Procedure for Determining Moisture Content in Interior Wall Paint Using Karl Fischer Method
- Testing of Color Fastness to Rubbing for Textiles
- Qualitative Identification of Textile Fibers by Five Experimental Methods
- Tablet Dissolution Test - Determination of Dissolution Rate and Dissolution Rate of Azithromycin Dispersible Tablets
- Determination of Chemical Oxygen Demand (COD) in Water - Potassium Dichromate Method
- How to Detect Bacteria in Food: Total Bacterial Count Procedure
- Testing Coliforms in Food: MPN & Plate Count Methods
- Determination of Aflatoxin B1 in Food: ELISA & TLC Methods for Accurate Detection
- Determination of Molds and Yeasts in Food: Plate Count Methods for Accurate Detection
- Determination of Lubricating Oil Liquid Density - Density Meter Method
- Determination of Acid Value in Lubricating Oil - Potentiometric Titration Method
- ELISA Experimental Procedure for Detecting Aflatoxin B1 in Peanuts
- Careers
- About
- Contact
- Home
- Industries
- Food Testing
- Coffee Testing
Coffee Testing
InquiryThrough our global network of testing experts and analytical equipment including chromatography (HPLC, GC, GC/MS) and atomic absorption spectroscopy (AAS, GFA, FIAS), Our goal is to provide test services as efficiently as possible to maximize our customers' profits. For more information about our services, contact one of our experts today.
Note: this service is for Research Use Only and Not intended for clinical use.
The US Food and Drug Administration (FDA) issues warnings to consumers about high concentrations of caffeine products. The FDA believes these products pose a threat to public health. The guidelines issued by the FDA clearly state that it is illegal to directly sell dietary supplements containing pure caffeine or high concentrations of caffeine in powder or liquid form. These products have a very high risk of misuse of dangerous doses, which caused at least two deaths. Caffeine is a powerful stimulant, and very small amounts of pure caffeine or high concentrations of caffeine can have serious or even fatal effects. Symptoms of overdose of caffeine include rapid heartbeat, tremors, vomiting, diarrhea, unstable walking and coma. The FDA is concerned that teenagers and young people who consume these products only understand its benefits and are not aware of the health risks of these products. The main ingredient in coffee is caffeine, and coffee has always been a popular drink which makes it necessary to conduct a safe test on coffee. As your trusted partner, Alfa Chemistry can provide you with a complete solution to support your business needs and accurate analysis to erase your worries about coffee in the most efficient way.

Alfa Chemistry's testing services for coffee include but not limited to:
Concentrations of Acrylamide
Coffee Bean Powder Moisture Content
Techniques for coffee testing
Coffee Grinding Machine
The coffee grinding machine is one of the most commonly used devices in coffee bean testing, used to grind coffee beans into different particle sizes for quality assessment.
Moisture Meter
The moisture meter measures the moisture content in coffee beans, helping to determine their storage time and whether they have been over-roasted.
Karl Fischer Moisture Titrator
The Karl Fischer moisture titrator is used to determine the moisture content in instant coffee, serving as an important indicator for evaluating instant coffee quality.
Fat Content Analyzer
The fat content analyzer tests the fat content in coffee, typically using the Soxhlet extraction method to extract crude fat from the coffee, in order to assess its quality.
Soluble Solids Meter
The soluble solids meter measures the soluble substances in coffee beans, including caffeine and other organic acids, facilitating the evaluation of coffee bean quality and flavor.

Industry articles
Multi-Element Determination in Green Coffee Beans
 de Gois, Jefferson S., Izylla O. Lucena, Paulo S. de O. Cezario, Arnaldo P. da Silva, Igor CA Lima, and Aderval S. Luna. Analytical Methods 10, no. 14 (2018): 1656-1661.
de Gois, Jefferson S., Izylla O. Lucena, Paulo S. de O. Cezario, Arnaldo P. da Silva, Igor CA Lima, and Aderval S. Luna. Analytical Methods 10, no. 14 (2018): 1656-1661.
This study describes the multivariable development of a simple method based on the use of dilute nitric acid and ultrasonic-assisted extraction (UAE), followed by determination using inductively coupled plasma optical emission spectrometry (ICP-OES). The method was applied to the multi-element determination of newly developed coffee varieties.
Extraction time and HNO3 concentration were optimized, achieving quantitative recovery with an extraction time of 15 minutes and 0.6 mol L-1 HNO3. After the extraction procedure, 0.48 mol L-1 HCl was added to stabilize the analytes in the dilute HNO3 solution. The accuracy of the method was assessed by comparing it with microwave-assisted digestion (MAD) and recovery tests. According to a t-test at a 95% confidence level, the results from UAE were consistent with those obtained from MAD.
A total of thirty-five coffee bean samples were analyzed using the proposed method. The results indicated that the concentrations of various elements were as follows: Ba ranged from 0.2 to 4.5 μg g-1, Ca from 1507 to 2576 μg g-1, Cu from 5 to 30 μg g-1, Fe from 17 to 44 μg g-1, K from 30,771 to 55,741 μg g-1, Mg from 2005 to 3327 μg g-1, Mn from 12.1 to 148.8 μg g-1, Sr from 1.0 to 7.6 μg g-1, P from 1105 to 2766 μg g-1, and Zn from 4 to 12 μg g-1.
Determination of Caffeic Acid in Turkish Coffee
 İNCEBAY, Hilal. Journal of Food Composition and Analysis (2024): 106717.
İNCEBAY, Hilal. Journal of Food Composition and Analysis (2024): 106717.
Caffeic acid possesses anti-inflammatory, antimicrobial, anticancer, and immunomodulatory properties, along with antioxidant characteristics. Therefore, accurately determining the content of this acid is important for analytical and therapeutic purposes, as well as for the safety and quality of food samples.
This study aims to determine the content of caffeic acid in Turkish coffee using square wave voltammetry. The method has been successfully applied for the precise determination of caffeic acid, utilizing a glassy carbon electrode modified with a synergistic combination of functionalized multi-walled carbon nanotubes and boron oxide nanoparticles. The proposed platform and method achieved a limit of quantification of 0.04 nM for caffeic acid within a dynamic range of 1.0 nM to 40 μM, and were successfully used for the determination of caffeic acid in Turkish coffee samples.
Determination of Phthalates and Di(2-ethylhexyl) Adipate in Coffee Capsules
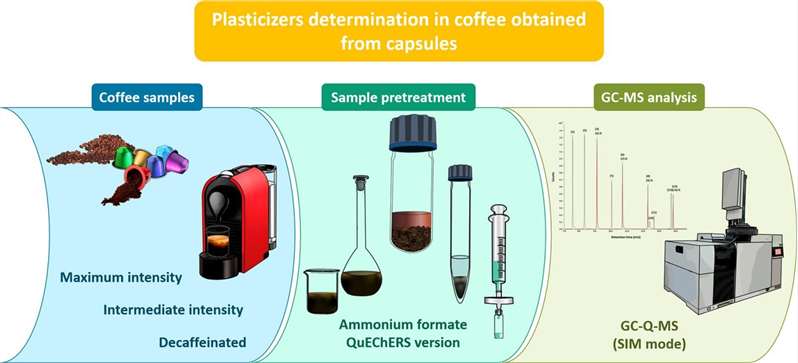 Domínguez-Hernández, Cristopher, et al. Food Chemistry 388 (2022): 132997.
Domínguez-Hernández, Cristopher, et al. Food Chemistry 388 (2022): 132997.
The ammonium formate version of the QuEChERS method was applied for the first time to extract a set of nine phthalates and one adipate from three types of coffee prepared from coffee capsules (high concentration, medium concentration, and decaffeinated), using gas chromatography coupled with mass spectrometry for the separation and determination of analytes. Matrix-matched calibration showed that the determination coefficients (R2) for all analytes and the matrix were above 0.9983, indicating good linearity. Overall, the assessment of matrix effects revealed moderate signal suppression, while the average relative recovery values for most analytes ranged from 70% to 120%, with relative standard deviation values of ≤19%. Several samples of each coffee type obtained from capsules made of different materials were also analyzed, revealing concentrations of DBP, DEHA, and DEHP in the range of 29.3 to 734 ng/capsule, which are below the established acceptable daily intake levels for some of these samples.
Analysis of Perchlorate and Chlorate in Coffee Samples
 Zhang, H., Feng, X., Liu, D., Wang, X., Wei, J., & Liu, H. (2022). Microchemical Journal, 181, 107822.
Zhang, H., Feng, X., Liu, D., Wang, X., Wei, J., & Liu, H. (2022). Microchemical Journal, 181, 107822.
A liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was established using a tri-functional modified hydrophilic interaction liquid chromatography (HILIC) column to measure chlorate and perchlorate in instant coffee and ready-to-drink (RTD) coffee.
After sample extraction and purification using an MCX column, separation was performed on a Tour DEA column (100 mm x 2.1 mm) with a gradient program employing 0.4% ammonia - 2 mmol/L ammonium acetate solution and acetonitrile as the mobile phase. To ensure the reliability and sensitivity of the method, isotopic internal standards and a multiple reaction monitoring (MRM) model were utilized during quantification. Satisfactory data were obtained during the validation process, with linear ranges for chlorate and perchlorate being 5.0 to 1000 μg/L and 0.5 to 100 μg/L, respectively. The recovery rates for chlorate in coffee samples ranged from 98.7% to 115%, while the recovery rates for perchlorate ranged from 92.3% to 102%, with relative coefficients being satisfactory (<10%). The limits of quantification for chlorate and perchlorate were 5 μg/L and 0.5 μg/L, respectively.
Determination of Ochratoxin A in Coffee Beverages
 Cina, Mariel, et al. Journal of Food Composition and Analysis 114 (2022): 104777.
Cina, Mariel, et al. Journal of Food Composition and Analysis 114 (2022): 104777.
Ochratoxin A (OTA) is a secondary metabolite produced by filamentous fungi and is classified as Group 2B by IARC. OTA is present in various foods and beverages.
This study developed and validated a new method for the detection and quantification of OTA in coffee drinks. The extraction procedure is based on dispersive liquid-liquid microextraction and solidification of floating organic droplets (DLLME-SFO). The extracts were analyzed using UHPLC-(ESI+)-MS/MS. The method demonstrated high sensitivity, with limits of detection (LOD) and quantification (LOQ) of 0.3 and 0.9 ng/mL, respectively, and a linear range of 0.3 to 70 ng/mL, with a correlation coefficient (R2) of 0.9962. The relative standard deviation ranged from 1.1% to 8.7% (n = 3). Additionally, satisfactory extraction recovery rates were obtained, averaging 88.3%, with an enrichment factor of 7.
Coffee test guidance, standards and specifications
FDA
- Regulatory Resources
- Application Notes
- Blog
- Recommendation
- Ordering Process
- Ask a Question
Do not know how to place an order, please refer to the flow chart shown below.
Submit quotation request |
A technical manager will contact you within 24 hours |
You will review and approve the final price and place an order |
Confirm with you and make the payment |
Instruct you to ship your samples and form |
Analytic report delivery |


