It is oligonucleotide therapeutics that have changed modern medicine, providing specialized treatments for previously incurable conditions. They are drugs - artificial RNA or hybrid RNA-DNA molecules that control RNA activity or protein levels. Their exact workings have made it possible to cure rare genetic diseases and medically underserved conditions.
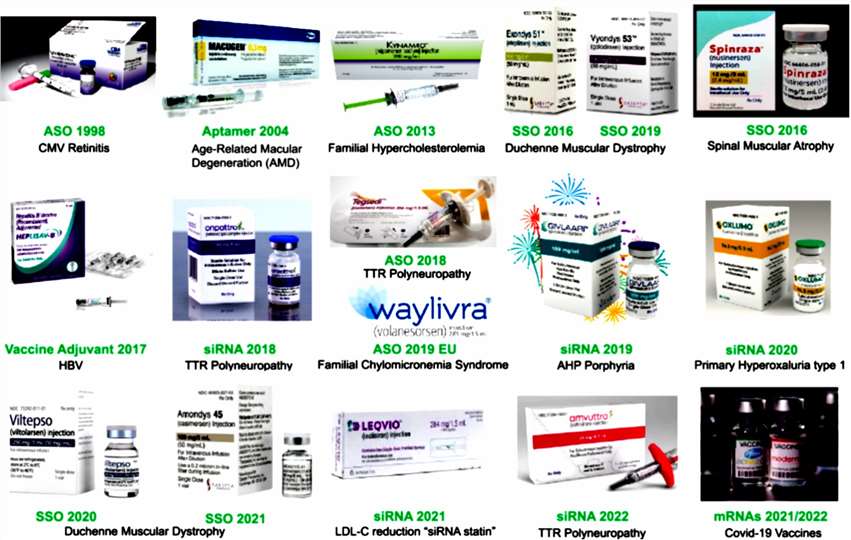 Figure 1. Currently approved oligonucleotide drugs and mRNA vaccines[1].
Figure 1. Currently approved oligonucleotide drugs and mRNA vaccines[1].
Growth of FDA Approvals
There have been 20 oligonucleotide drugs approved by the FDA since 1998, and it's climbed to roughly two new drugs every year since 2016. Such approvals range from antisense oligos (ASOs) to small interfering RNAs (siRNAs) to aptamers, reinforcing the multidisciplinary nature of this class of drugs.
Here we have listed FDA-approved classes of oligonucleotides.
Antisense Oligonucleotides (ASOs)
ASOs were the first class of oligonucleotide medications to be approved by the FDA. Such molecules of single-stranded DNA or RNA attach to complementary RNA targets, and influence gene expression by either RNA cleavage or translation inhibition.
Small Interfering RNAs (siRNAs)
siRNAs shut down specific gene expressions by knocking down target mRNA. They promise to cure genetic and chronic diseases.
Aptamers
Aptamers are oligo-dimers that have one or more single-stranded strands that bind with excellent fidelity to molecular targets. Fewer aptamer drugs are currently available, but they still have a lot of potential in precision medicine.
Table 1: FDA-approved oligonucleotide drugs
| Type | Drug | CAS | Indications | Year of FDA Approval |
| ASO | Fomivirsen | 144245-52-3 | Cytomegalovirus retinitis in an immunocompromised patient. | 1998.08 |
| ASO | Mipomersen | 1000120-98-8 | Familial hypercholesterolemia in a purebred. | 2013.01 |
| ASO | Nusinersen | 1258984-36-9 | Spinal muscular atrophy. | 2016.12 |
| ASO | Eteplirsen | 1173755-55-9 | Duchenne muscular dystrophy. | 2016.09 |
| ASO | Volanesorsen | 915430-78-3 | Familial chylomicronaemiasyndrome (FCS), also known as type I hyperlipoproteinemia. | 2019.05 |
| ASO | Defibrotide | 83712-60-1 | Veno-occlusive disease. | 2016.03 |
| ASO | Inotersen | 1492984-65-2 | Hereditary transthyretin-mediated amyloidosis. | 2018.01 |
| ASO | Golodirsen | 1422959-91-8 | Duchenne muscular dystrophy. | 2019.12 |
| ASO | Viltolarsen | 2055732-84-6 | Duchenne muscular dystrophy. | 2020.08 |
| ASO | Casimersen | 1422958-19-7 | Duchenne muscular dystrophy. | 2021.02 |
| ASO | Eplontersen | 1637600-16-8 | ATTRv-PN. Hereditary Transthyretin Amyloidosis | 2023.12 |
| siRNA | Patisiran | 1420706-45-1 | Hereditary transthyretin-mediated amyloidosis. | 2018.08 |
| siRNA | Givosiran | 1639325-43-1 | Acute hepatic porphyria. | 2019.11 |
| siRNA | Lumasiran | 1834610-13-7 | Primary hyperoxaluria type 1. | 2020.11 |
| siRNA | Inclisiran | 1639324-58-5 | Primary hypercholesterolemia. | 2021.12 |
| siRNA | Vutrisiran | 1867157-35-4 | Hereditary transthyretin-mediated amyloidosis. | 2022.06 |
| siRNA | Nedosiran | 2266591-83-5 | Primary Hyperoxaluria (PH) | 2023.09 |
| Aptamer | Pegaptanib | 222716-86-1 | Neovascular (wet) age-related macular degeneration. | 2004.12 |
Technological Advancements and Challenges
Recent efforts such as the European Pharma Oligonucleotide Consortium (EPOC) are trying to standardize manufacturing processes for oligonucleotide drugs. Among its focus areas are faster development for first-in-human clinical trials, more drug product sterilisation and higher quality standards.
Solving oligonucleotide drug problems consists in getting them to deliver more effectively to the targeted tissues and keep them stable against enzymatic degradation. These problems have been greatly ameliorated by developments in lipid nanoparticles (LNPs) and chemical modifications like 2'-O-methyl and phosphorothioate backbones.
 Figure 2. Multifunctional Ti3C2 MXene-based nanocomposite (HA-Ti3C2@ASO/DOX) for simultaneous delivery of antisense oligonucleotides and chemotherapeutic drugs[4].
Figure 2. Multifunctional Ti3C2 MXene-based nanocomposite (HA-Ti3C2@ASO/DOX) for simultaneous delivery of antisense oligonucleotides and chemotherapeutic drugs[4].
Therapeutic Potential and Future Directions
The precision for rare and chronic diseases is unsurpassed by oligonucleotide treatments. They are being used in personalized and precision medicine at an exponential rate. Current trends include:
a. Building more stable delivery vehicles, like conjugated peptides and LNPs.
b. Expanding indications beyond rare disorders to common ones such as cancer and metabolic disease.
c. Regulatory authorities working with pharmaceutical firms to align development activities.
Conclusion
The FDA's growing list of approved oligonucleotide drugs highlights the therapeutic and commercial viability of these groundbreaking treatments. As molecular targeting and production become even better, oligonucleotide treatments will become ever more central to our medical lives.
Related Products & Services
References
- Egli M., et al. Chemistry, Structure and Function of Approved Oligonucleotide Therapeutics. Nucleic Acids Research . 2023, 51(6), 2529-2573.
- Vinjamuri BP., et al. A Review on Commercial Oligonucleotide Drug Products. Journal of Pharmaceutical Sciences. 2024, 113(7), 1749-1768.
- Stein Cy A., et al. FDA-Approved Oligonucleotide Therapies in 2017. Mol Ther. 2017, 25(5):1069-1075.
- He L., et al. Antisense Oligonucleotide Facilitate the Photothermal Therapy of Mxene-based Nanoplatform for Drug-resistant Breast Cancer. Materials Today Communications. 2024, 41, 110579.
Our products and services are for research use only and cannot be used for any clinical purposes.



 Figure 1. Currently approved oligonucleotide drugs and mRNA vaccines[1].
Figure 1. Currently approved oligonucleotide drugs and mRNA vaccines[1]. Figure 2. Multifunctional Ti3C2 MXene-based nanocomposite (HA-Ti3C2@ASO/DOX) for simultaneous delivery of antisense oligonucleotides and chemotherapeutic drugs[4].
Figure 2. Multifunctional Ti3C2 MXene-based nanocomposite (HA-Ti3C2@ASO/DOX) for simultaneous delivery of antisense oligonucleotides and chemotherapeutic drugs[4].