 MH van der Ree, et al. The Lancet, 2017, 38(10070), 709-717
MH van der Ree, et al. The Lancet, 2017, 38(10070), 709-717
RG-101 represents a groundbreaking approach to managing chronic hepatitis C virus (HCV) infection by targeting host microRNA-122 (miR-122), an essential factor for HCV replication.
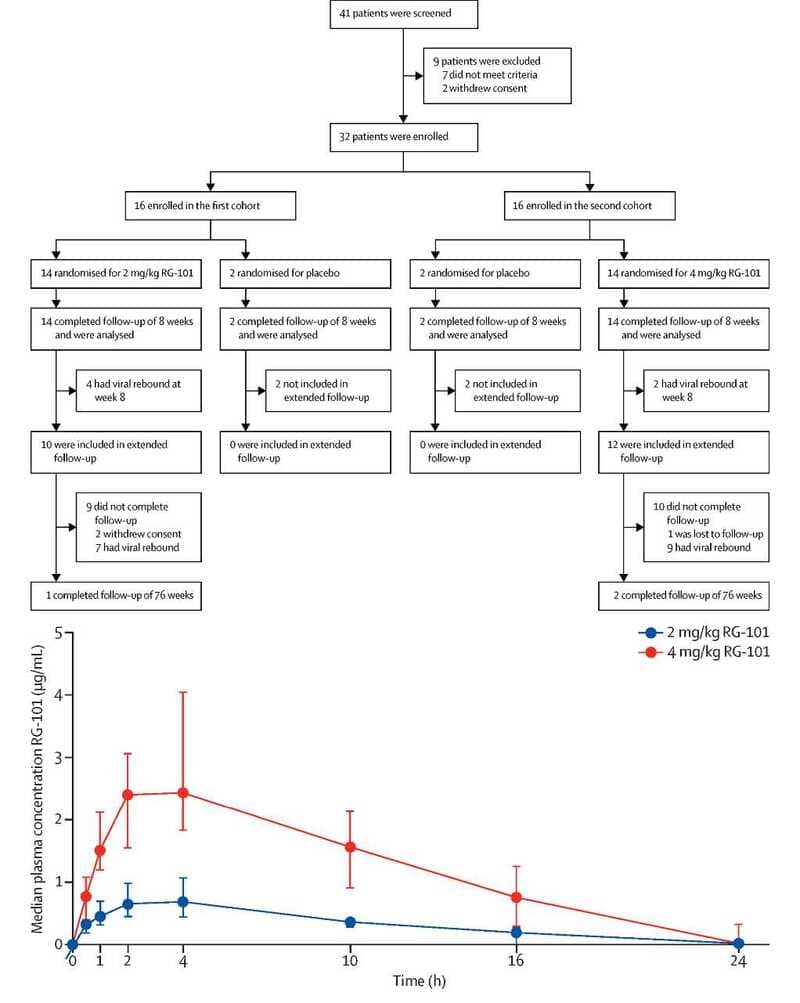
In a phase 1B, randomized, double-blind trial, RG-101 was administered as a single subcutaneous injection at doses of 2 mg/kg or 4 mg/kg to 32 patients with chronic HCV genotypes 1, 3, or 4. This innovative therapy demonstrated robust antiviral activity, with a median viral load reduction of 4.42 log10 IU/mL and 5.07 log10 IU/mL for the respective doses by week 4. Importantly, three patients maintained undetectable HCV RNA levels for 76 weeks post-administration, showcasing its potential for sustained virological response (SVR).
RG-101's mechanism of action involves antagonizing miR-122, a microRNA crucial for stabilizing and facilitating the translation of the HCV genome. By binding miR-122, RG-101 disrupts the HCV replication cycle, leading to rapid and profound reductions in viral load. Notably, viral rebound observed in some patients was linked to resistance-associated substitutions in miR-122 binding sites within the 5' UTR of the HCV genome.
The treatment was well tolerated, with 26 of 28 RG-101 recipients reporting mild-to-moderate adverse events. The absence of serious safety concerns supports its potential as a feasible therapeutic option.



 MH van der Ree, et al. The Lancet, 2017, 38(10070), 709-717
MH van der Ree, et al. The Lancet, 2017, 38(10070), 709-717